(SHTT) – The World Health Organization (WHO) has recently issued a warning that four cough and cold syrups from Indian pharmaceutical company Maiden have the potential to cause acute kidney damage. even lead to the risk of death for children.
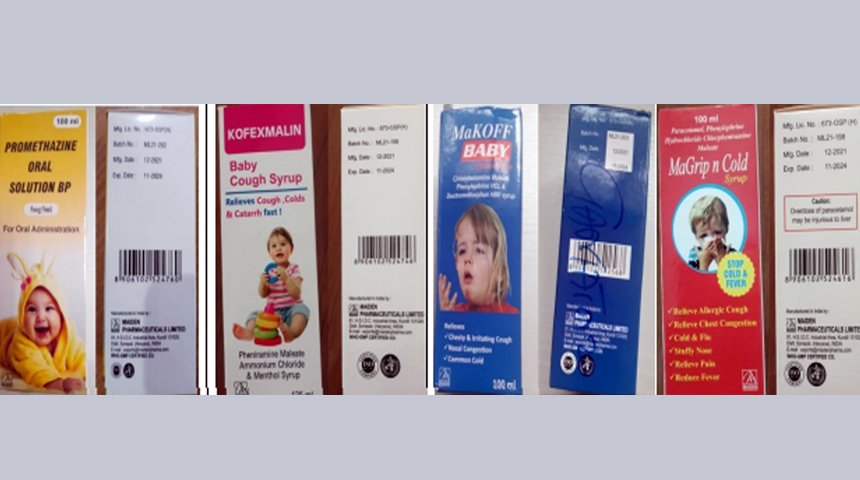
According to information published on October 5, 2022, the World Health Organization said that 4 types of products suspected of causing health hazards include: Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, Magrip N Cold Syrup.

According to the announcement of the WHO representative at the press conference on October 5, the analysis results of the four above-mentioned cough syrups and medicines showed the presence of “unacceptable” diethylene glycol and ethylene glycol. These are all identified as substances that are toxic to humans when used and pose a risk of death.
WHO also warns Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, Magrip N Cold Syrup can cause acute kidney damage. These products have also been confirmed to be the cause of death for 66 children in Gambia.
Some other cases of using the products warned may be subject to milder side effects such as: abdominal pain, vomiting, diarrhea, urinary retention, headache, mental confusion.
Maiden only manufactures and exports the cough syrup to the Gambia, sources with the Indian Ministry of Health said. Maiden announced on its website that it has two manufacturing plants in Kundli and Panipat in Haryana state, both near New Delhi, and has recently built another.
Maiden’s annual production capacity is 2.2 million bottles of syrup, 600 million capsules, 18 million injections, 300,000 ampoules of ointment and 1.2 billion tablets. Maiden sells its products domestically and exports to countries in Asia, Africa and Latin America.
According to the Indian Ministry of Health, importing countries often test medicinal products before licensing them for use.
WHO added that Maiden’s cough syrup products including Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup may have been distributed elsewhere through unofficial markets. but has only been identified in the Gambia so far.
Because of its widespread distribution, WHO also issued an additional warning about possible global exposure. Currently, WHO calls on countries to review and ban these products so as not to cause further harm to patients.
Investigations into the pharmaceutical company Maiden and the drug regulatory authorities in India are currently being urgently conducted by WHO and Indian authorities.
The authorities are also warning consumers if they have purchased products including Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup not to use.
In the event that you or those around you have used products related to warning information and appear abnormal health signs, people should immediately go to medical facilities to check and receive advice. Get medical advice as soon as possible from a medical professional.
Thai An










